On June 7, 2021, the US Food and Drug Administration (FDA) approved the first new drug therapy for Alzheimer’s disease since 2003. The drug, aducanumab, was developed by pharmaceutical companies Biogen and Eisai, and will be sold under the brand name Aduhelm.
Unlike previously approved drugs, aducanumab aims to slow the biological progression of Alzheimer’s disease, rather than just easing symptoms. However, aducanumab is not a cure for Alzheimer’s — nor can it reverse disease progression.
Despite the excitement prompted by a new Alzheimer’s drug on the market, the FDA’s decision remains controversial.
How aducanumab works in the brain
Aducanumab is a type of antibody, and it targets clumps of amyloid-beta protein that accumulate between brain cells during Alzheimer’s disease. These amyloid-beta plaques stop brain cell function, contributing to cognitive issues and decline.
The FDA approved aducanumab mainly on the basis that it can reduce amyloid plaques in the brain.
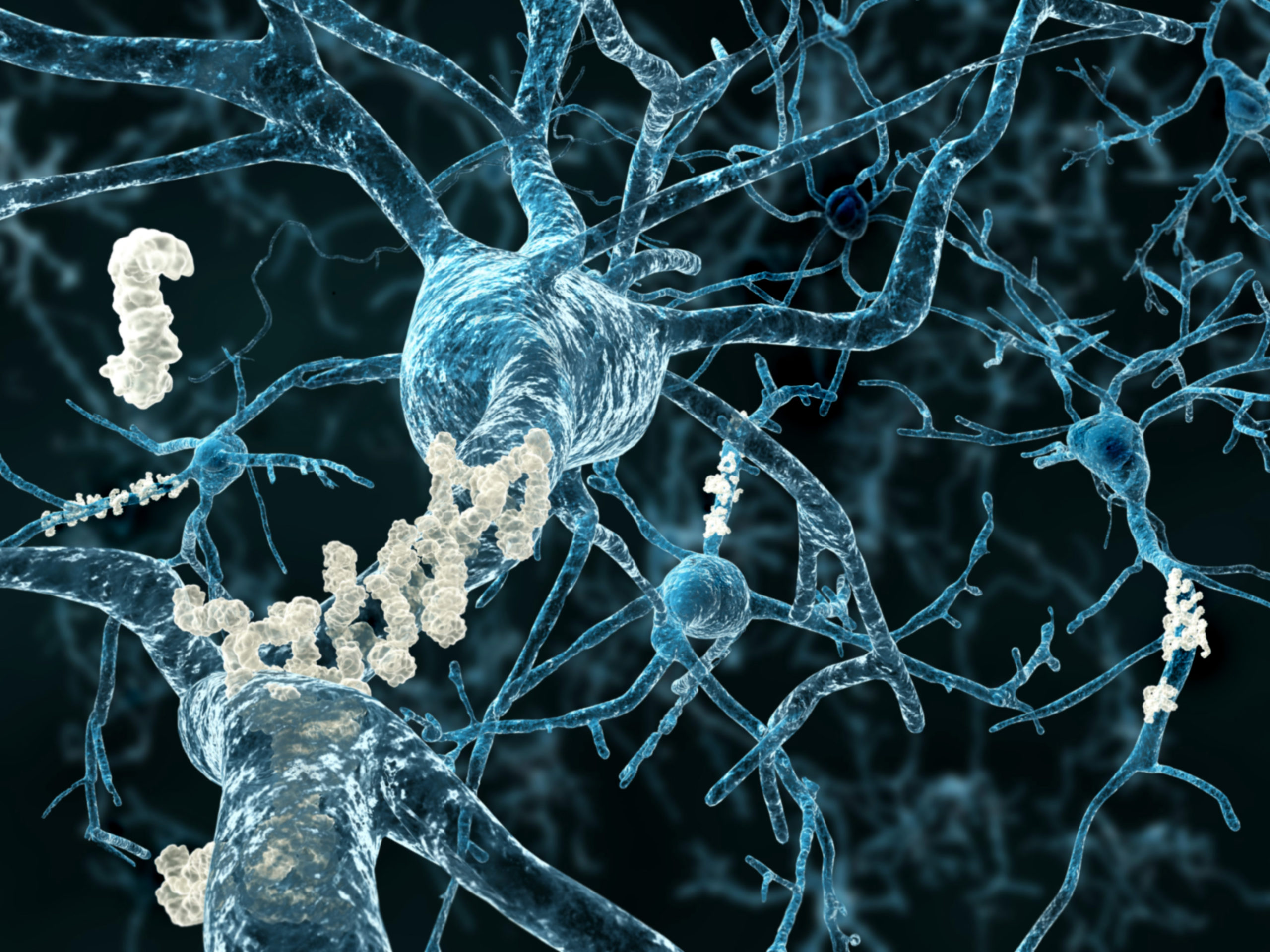
Amyloid plaques forming around neurons during Alzheimer’s disease.
Because Alzheimer’s disease is so complex, and its causes uncertain, eliminating plaques does not necessarily mean preventing cognitive decline. These uncertainties give rise to some of the disagreement surrounding aducanumab.
Why is aducanumab’s approval so contentious?
The FDA approved aducanumab via accelerated approval—a process reserved for drugs to treat serious or life-threatening illnesses. The drugs must confer benefits over existing treatments. Alzheimer’s afflicts nearly 6 million Americans; the disease’s consequences ripple throughout families and support networks, and across the global community. The need for effective treatments is clear and urgent.
But is aducanumab the right treatment? An expert advisory panel urged the FDA to reject the drug due to a lack of evidence for its effectiveness.
“The treatment is controversial among the experts,” said Dr. Mike Weiner, the Principal Investigator of the Brain Health Registry. “The best interpretation of the clinical trial data is that it slows decline by about one third. Some people receiving the drug will probably show a strong benefit and others no benefits.” Furthermore, aducanumab has side effects including brain swelling, but serious side effects occur in only a very small fraction of those treated.
The drug is also expensive, totaling an annual cost of about $56,000. PET scans may be required prior to receiving this drug, and multiple MRIs are recommended to monitor side effects. As of now, it is unclear whether or not Medicare will pay for these scans and for the drug.
Moving forward, the FDA will require post-approval clinical trials to continue to monitor the drug’s effects.
Regardless of whether or not aducanumab works, the FDA’s decision has implications for the Alzheimer’s field moving forward, and for the evaluation of future drug therapies.
“At this point, it’s unclear to what extent aducanumab is going to be impactful in the population,” Dr. Weiner said. “We just need much more research. One way research in this area can progress is by having more people join research studies like BHR.”
BHR is designed to help other researchers recruit for their studies, including Alzheimer’s disease clinical trials.




