Haga clic aquí para leer este boletín en español.
Despite the ongoing Covid-19 pandemic, 2021 was a groundbreaking year for both the Brain Health Registry (BHR) and for the greater Alzheimer’s research field. As we reflect on the past 12 months, we’d like to share highlights from both BHR and from the field as a whole.
Drug Discovery
In June 2021, the US Food and Drug Administration approved the drug Aduhelm (aducanumab) for the treatment of Alzheimer’s disease. Unlike previously approved drugs, which can help manage disease symptoms, Aduhelm was developed to slow the biological progression of Alzheimer’s disease.
The drug specifically targets the sticky plaques of amyloid-beta protein that form between brain cells. Amyloid plaques contribute to brain changes leading to impaired memory and thinking.
Additional drugs developed to eliminate amyloid plaques have shown promise in clinical trials. For example, in a study of Eli Lilly’s donanemab, the drug reduced amyloid in the brain for two thirds of trial participants. Additionally, trial participants who received the drug experienced cognitive decline at a rate 32% slower than participants who did not receive treatment.
Similar drugs are being developed and tested by pharmaceutical companies Eisai and Roche.
Researchers are also investigating other types of drugs that target aspects of Alzheimer’s pathology besides amyloid plaques. These targets include the gut microbiome — the trillions of fungi, bacteria, and other microbes inhabiting your gut — another protein called tau, and inflammation in the brain.
Covid-19
Although Covid-19 has profoundly disrupted many research studies, it has also accelerated development and use of remote assessment tools for cognition. The BHR has been a major innovator in this area, and our research has continued to move forward. Thanks to all who participate in BHR!
Blood Tests
Blood tests for Alzheimer’s disease have the potential for easy, routine use in a doctor’s office. These tests have been shown to change in response to treatments which remove amyloid plaques.
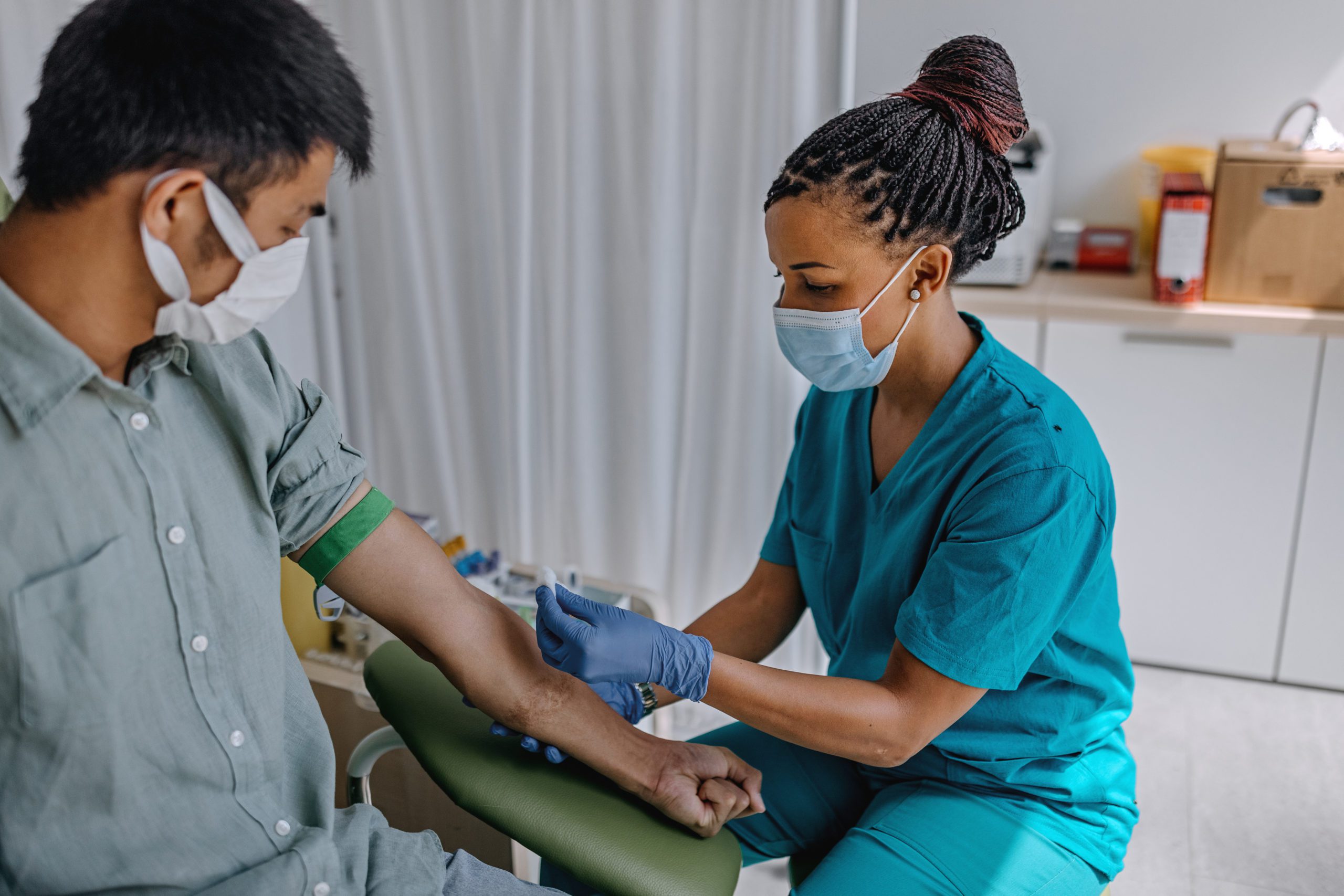 Blood samples contain substances that can provide valuable information about a person’s likelihood of developing Alzheimer’s disease. Furthermore, they are less invasive and expensive than brain imaging methods like Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET), which are typically used to help diagnose brain diseases.
Blood samples contain substances that can provide valuable information about a person’s likelihood of developing Alzheimer’s disease. Furthermore, they are less invasive and expensive than brain imaging methods like Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET), which are typically used to help diagnose brain diseases.
The BHR used our platform to involve some participants in a blood collection study. Read more about that study in our December 2020 Newsletter.
Diversity in Alzheimer’s Disease Research
The importance of diversity in clinical research and clinical trials has received increasing emphasis and attention throughout the research community. Having study participants that represent the entire population is important for developing effective treatments that can benefit everyone.
The BHR has pioneered a series of initiatives to recruit underrepresented communities including Black and Latino/a/x adults. These projects include the CEDAR study, which is aimed at increasing engagement by our Black participants, and our Latino Brain Health Registry.

—
As we look back on all these achievements, we are extremely excited for what 2022 has in store. As always, we are grateful for all our participants that make this research possible. Happy New Year from everyone on the Brain Health Registry team!




