Comprehensive referral programs function under both UCSF’s IRB and the collaborator’s IRB. Collaborator’s IRB should be amended to include BHR as a recruitment source. Collaborator IRB protocol should also include that site staff will provide information about BHR participants who make contact with the site, including information on interest level, eligibility, and enrollment in the referral study. BHR can provide the collaborator with the following materials: a) copy of redacted BHR protocol; b) suggested text that describes BHR and identifies it as a recruitment source; c) templates for emails and any other participant communication; and d) any other additional documents required by the collaborator IRB.
Download Referral Email Template
Download Study Description
Download Data Sharing Consent
Program Add-on:
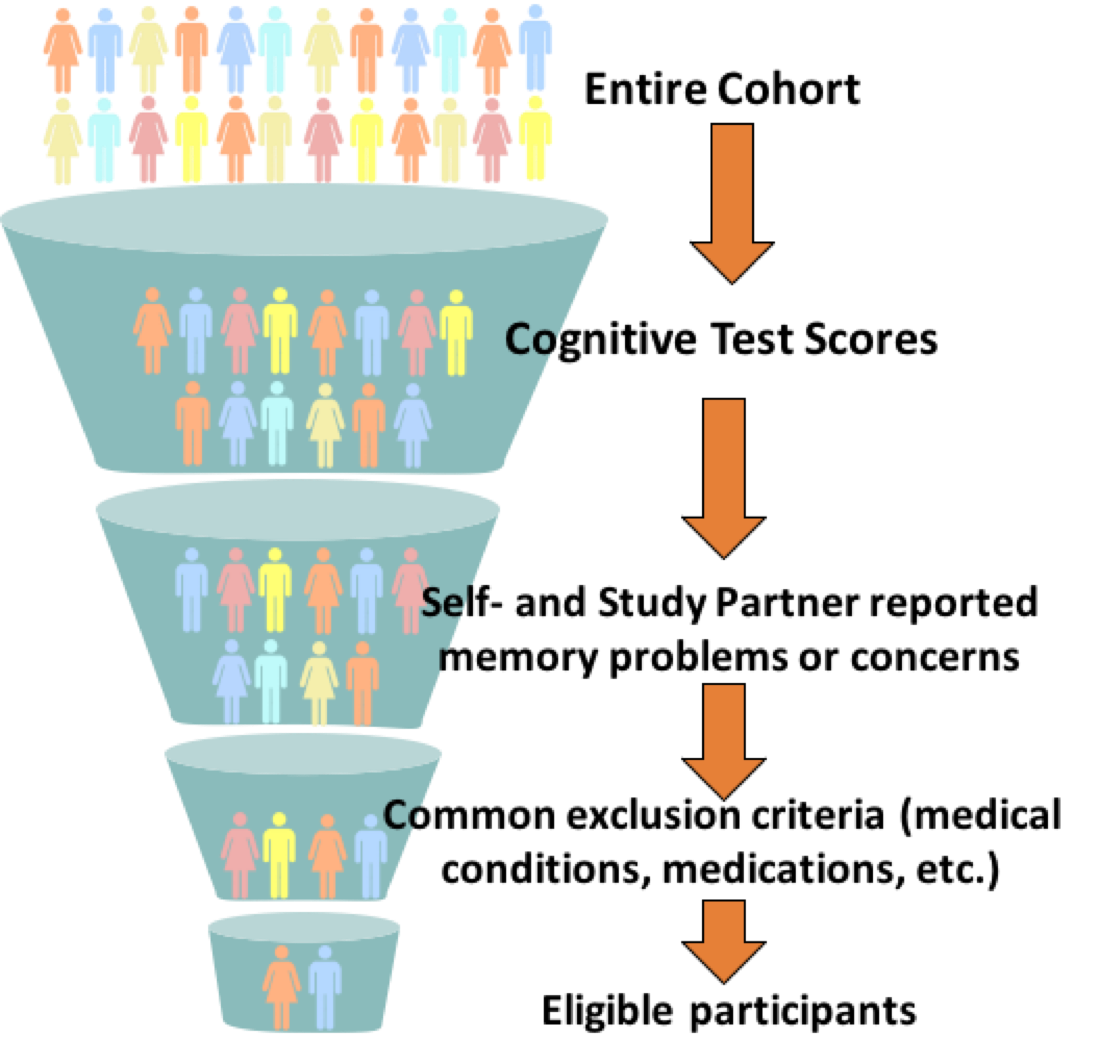
Additional screening:
Upon collaborator request, additional screening for comprehensive referrals can be collected by BHR through the use of other detailed questionnaires and/or cognitive data at one or more visits. This allows a study to use their own specific inclusion/exclusion criteria and site location information to identify a potentially more suitable referral.
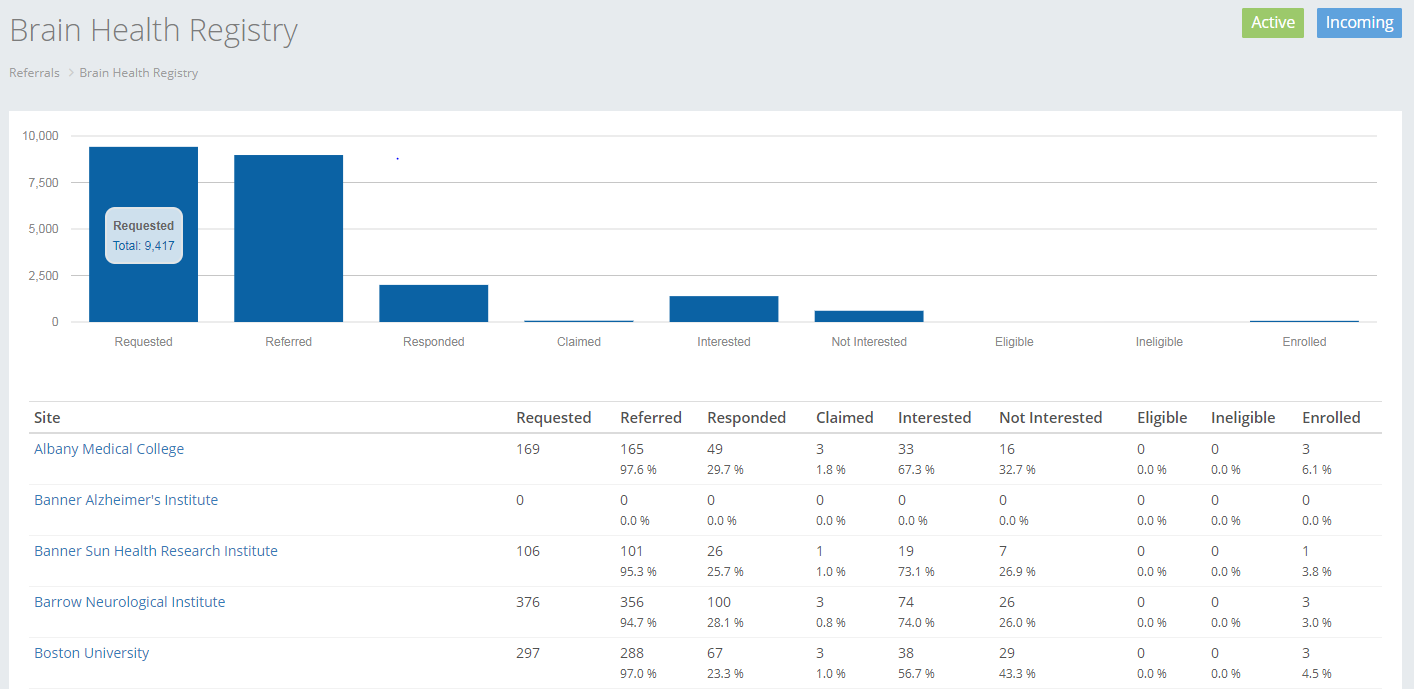
BHR data on referred participants:
Existing BHR data collected on referred participants can be provided to the collaborator. The BHR participants would need to complete a data sharing consent prior to any data being shared. A data dictionary and dataset would be generated and shared on a pre-determined schedule.
Next Steps:
If you are interested in a Comprehensive Referral Program, please complete the Comprehensive Referral Request Form and return the completed form to BHR.
Download Comprehensive Referral Request Form