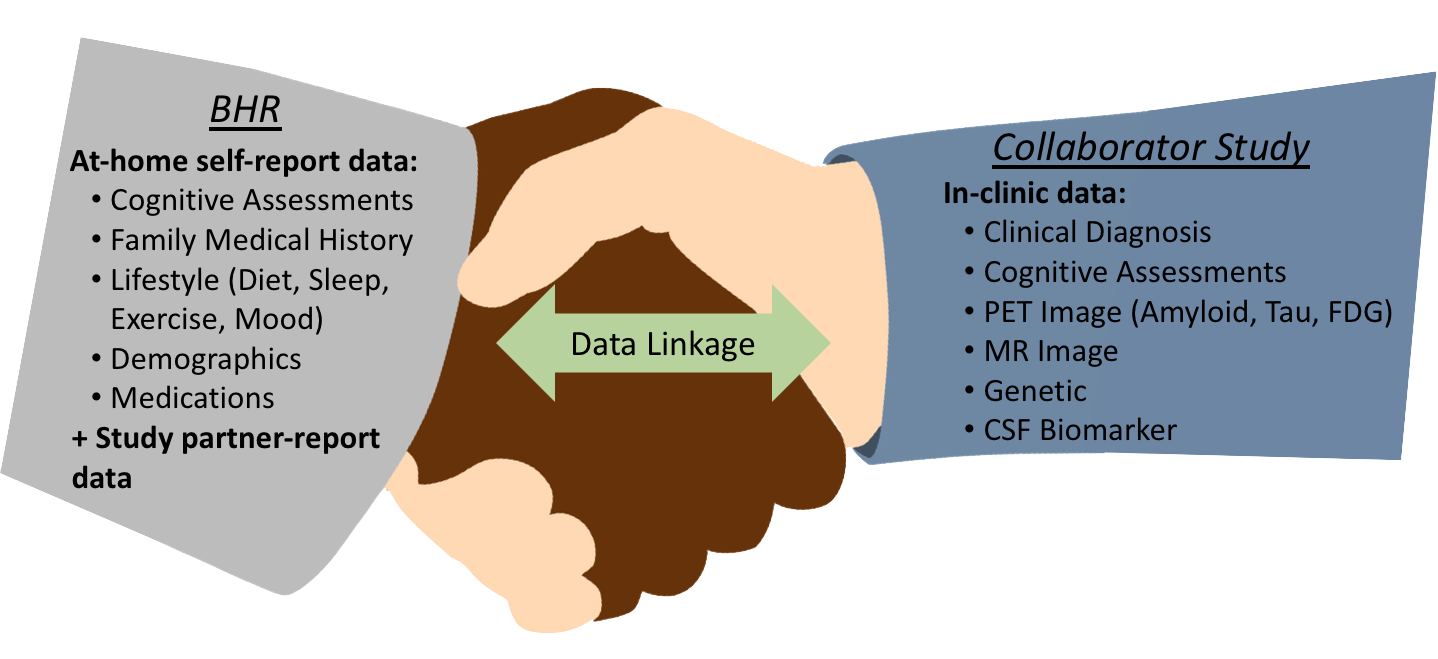
BHR will create a demo study in the co-enrollment portal including: a) co-branded login and registration pages; b) customized layout with task order, grouping and introductions; c) schedule of study visits; and d) co-branded invitation emails and study emails.
Collaborator will test the participant experience in the demo environment. Upon Collaborator feedback and approval, the final co-enrollment portal will be generated and launched in production.
Co-enrollment programs function under both UCSF’s IRB and the Collaborator’s IRB. BHR can provide collaborators with the following materials: a) copy of redacted BHR protocol; b) co-enrollment study design details; c) templates for emails and registration pages; d) suggested recruitment materials and e) any other additional documents required by the collaborator’s IRB.
Caregiver and Study Partner Portal (CASPP) Add-on:
Collaborators can chose to include the Caregiver and Study Partner Portal (CASPP) into their co-enrollment study. CASPP allows a study partner (SP) of a BHR participant to separately register, consent, and complete questionnaires. The data linked between the SP and participant includes six questionnaires, each of which takes approximately 3-10 minutes to complete. The questionnaires can be broadly characterized as gathering information about the participant and about the SP him/herself. Questions about the participant include a short health screener, ECog and FAQ adapted for online use, and questions about effective symptoms and disruptive behaviors associated with brain illness. Questions about the SP him/herself include demographics, a short health screener, stress, and SP relationship to participant. SP’s who identify as caregivers also complete the Caregiver Experience questionnaire within the CASPP. Please email [email protected] to receive a copy of the current questionnaires.